
Evaluation of efficacy and safety of Liv.52 DS tablets in acute viral hepatitis:
A prospective, double-blind, randomized, placebo-controlled,
phase III clinical trial
Dr. Rajiv Baijal, M.D., D.N.B.1, Dr. Nikhil Patel, M.D.,2 and Dr. S. A. Kolhapure, M.D.3* 1Gastroenterologist, 2Research Associates, Department of Gastroenterology,
Jagjivanram Western Railway Hospital, Mumbai Central, Mumbai, India. 3Senior Medical Advisor, R&D Center, The Himalaya Drug Company, Bangalore, India
Liv.52 DS tablets in acute viral hepatitis
|
Prior to the discovery of HCV, hepatitis following blood transfusion that was not caused by hepatitis A or hepatitis B was referred to as non-A, non-B hepatitis. Hepatitis C virus is a single-stranded RNA virus in the flaviviridae family. Primarily blood, blood products and sexual transmission transmit the virus (perinatal transmission is relatively low). About 85% of individuals acutely infected with HCV become chronically infected and most instances of acute infection are clinically undetectable. The natural history of chronic HCV infection can vary dramatically between individuals. About 20% of individuals with hepatitis C develop cirrhosis, which leads to end-stage liver disease; and there is an increased risk of developing hepatocellular carcinoma. History, serological testing and liver biopsy make the diagnosis of chronic hepatitis C. The presence of anti-HCV antibodies in a person with a risk factor or evidence of liver disease suggests the diagnosis of chronic hepatitis C. Tests for HCV RNA in blood in those individuals with anti-HCV antibodies confirms the diagnosis. The HDV (also called delta virus) is a small circular RNA virus and in humans, HDV infection only occurs in the presence of hepatitis B infection, as the HDV is replication defective and therefore cannot propagate in the absence of another virus. Hepatitis D virus infection is transmitted by blood and blood products; and the risk factors for infection are similar to those for HDV infection. A patient can acquire co-infection with HDV and HBV; and a patient with hepatitis B can be infected with HDV at any time after acute hepatitis B virus infection (super-infection). Hepatitis D virus super-infection should be suspected in a patient with chronic hepatitis B whose condition suddenly worsens and a particularly aggressive acute hepatitis B infection could suggest hepatitis D co-infection. Co-infection or super-infection with HDV in a patient with hepatitis B is diagnosed by the presence of antibodies against the HDV and IgM antibodies indicate acute infection |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
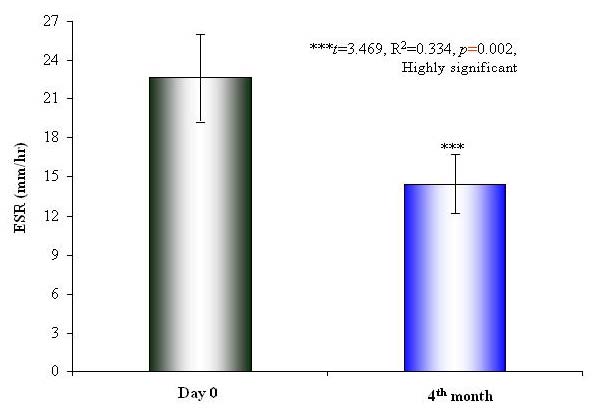
Hepatitis E Virus is very labile RNA virus with a particle diameter of 32-34 nm and it causes ET-NANBH, which is clinically indistinguishable from hepatitis A disease. Diagnosis of HEV is based on the epidemiological characteristics of the outbreak and by exclusion of hepatitis A and B viruses by serological tests. Hepatitis E virus is transmitted by the fecal-oral and person-to-person spread. Hepatitis E occurs in both epidemic and sporadic-endemic forms and the incubation period for hepatitis E varies from 2 to 9 weeks. The disease usually is mild and resolves in 2 weeks, leaving no sequelae. Pregnant women appear to be exceptionally susceptible to severe disease, and excessive mortality has been reported in this group. The HGV/HGBV-C is a recently characterized flavivirus (RNA virus) that may cause acute and chronic hepatitis. These new viruses are related to, but distinct from the flavivirus hepatitis C. Three viruses have been termed GB-A, GB-B and GB-C and based on genomic sequence comparisons; HGV is probably the same as GB-C. The precise role of HGV/GB-C in human disease is currently under investigation; however, most experts now feel that this virus is not responsible for clinically significant cases acute or chronic hepatitis.2-6 This study observed a highly significant and rapid symptomatic improvement in the mean scores for loss of appetite, weight loss, fatigue and jaundice in Liv.52 DS group, as compared to the placebo. There was a significant and rapid renormalization in the biochemical parameters of liver functions (SGOT, SGPT, SB), and the levels of SA, SG, and TP were renormalized. The increased levels of ESR and WBC were significantly reduced in Liv.52 DS group, as compared to the placebo group, at the end of the study. There were no clinically significant changes in other biochemical parameters such as Hb levels and PC, which indicate excellent short- and long-term safety profile of Liv.52 DS. There were no clinically significant adverse effects, either observed or reported; during the entire study period, and the |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

Copyrights © 2009 healthyliver.co.uk